Measuring Fuel Substrate Utilization: Indirect Calorimetry and RER
Above: Francis G. Benedict:
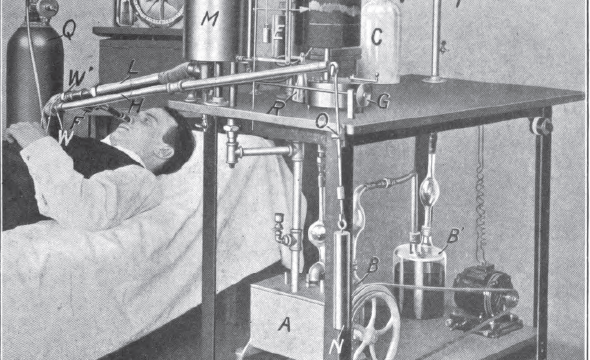
Francis G. Benedict was one of the world’s top scientists in the field of nutrition and metabolism. He was a member of the Society of American Magicians and performed professionally from 1938-1942. His investigations included mammals both domestic and wild, ranging in size from the 8-gram dwarf mouse to a 4000 pound elephant. Benedict conducted the original energy balance experiments on humans in 1906, pictured below.


Source readings: How and why I compare Mice, Men, Birds, Elephants
Respiration Exchange Ratio and the Chemistry of Combustion Illustrated
When we burn a particular fuel (e.g. glucose, fat, methane, propane) it ALWAYS combines with a fixed volume of O2 and produces a fixed volume of CO2.
The Chemistry of Combustion:
Methane: Cow Burps
CH4 + 2 O2 –> CO2 + 2 H2O
Glucose:
C6H12O6 + 6O2 –> 6 CO2 + 6H20
Fat:
C16H32O2 + 23O2 –> 16CO2 + 16H2O
As the formulas show – when a human cell aerobically uses 1 glucose molecule or 1 fat molecule – respectively:
- 6 CO2s get produced 6 O2s are consumed
- 16 CO2s get produced, 23O2s are consumed
RER for each:
- Glucose: 6CO2/6O2 = 1.
- Fat: 16CO2/23O2 = .7
Thus, RER ranges from .7 to 1
- When RER = 1, the body uses primarily glucose as fuel.
- An RER of .7 indicates the body uses primarily fat.
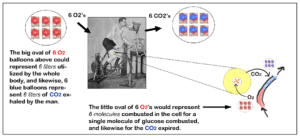
RER of Glucose Illustrated
Let’s assume the human on this bike burned ONLY glucose and nothing else. If it was, this is what we would see:
RER generally is close to 1 during high intensity exercise – when oxygen consumption is very high.
However, the body does not burn exclusively glucose or fat – but a mixture of the two, which is seen as RER changes with intensity level.
In order to complete this picture and know exactly the amount of fat and carbs burned at any intensity level – the RER of burning fat must be added – as illustrated here.
The red text shows the volume of oxygen used and the blue text shows the volume of carbon dioxide produced from combusting one molecule of each type of fuel.
The Chemistry of Combustion:
Methane:
CH4 + 2 O2 –> CO2 + 2 H2O
Propane
C3H8 + 5 O2 –> 3 CO2 + 4 H2O
Butane
C4H10 + 6.5 O2 –> 4 CO2 + 5 H2O
Glucose:
C6H12O6 + 6O2 –> 6 CO2 + 6H20
Fat:
C16H32O2 + 23O2 –> 16CO2 + 16H2O