Week 11. Visual the System, Aerobic Metabolism Part 1
IMPORTANT! Tap or click the image to dismiss or recover the caption.
If you seriously think about how an embryo gets oxygen into its body before using it, then you find yourself imagining the entire anatomical pathway oxygen takes within the oxygen delivery system before it is finally used at the cell level.
I purposely use the term oxygen delivery system as opposed to cardiovascular system, because it emphasizes flow or delivery of oxygen within your body before oxygen is used in a cell.
Thus I break this lecture into two sections: delivery of oxygen and usage of oxygen.
Section 1 Visualize the Oxygen Delivery System presents a gallery of visuals to picture the pathway oxygen takes from the heart to cells of the brain, muscles, and other tissues.
Section 2 The Root Knowledge ‘stops’ at the point where oxygen is used in a cell. At this point I present how aerobic metabolism is a matter of combustion of carbon – that is to say, the chemical process of combining oxygen with carbon in food – as it was classically taught in the early 20th Century.
Together, both sections present a concrete way of perceiving metabolism and a higher consciousness for eating the ‘right’ foods for health and human performance.
Section 2 in particular presents a transformative and more practical way to perceive food and metabolism by showing how vinegar or acetic acid is the body’s aerobic fuel. I think you will agree after learning how it works.
Let’s begin with the first section: visualize the body’s oxygen delivery system.
Section 1: Visualize the Oxygen Delivery System
- We want to visualize the entire structural pathway oxygen takes to get to your cells.
- The picture gallery breaks down the entire system.
IMPORTANT! Tap or click the image to dismiss or recover the captions.
The entire cardiovascular system is essentially like a garden hose attached to itself, but with a pump (the heart) attaching each end together.
It takes approximately 70 seconds for blood to return to the heart after freshly oxygenated blood exits the heart.
Now we proceed to the second objective of this lecture, which is to see how vinegar or acetic acid is the final aerobic fuel and the ultimate carbon ‘food chunk’ burned in a cell.
Section 2: The Root Knowledge
In a human, acetic acid’s 2 carbon atoms are utilized for aerobic metabolism.
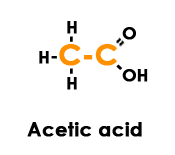
All food eaten – carbohydrates, protein, and fat must break down into acetic acid for a cell to use aerobically. Acetic acid is the one and only fuel burned.
Even ketones must be altered to acetic acid while on a low carb diet. So forget about any other way of trying to wrap your head around the subject; let’s get down to learning the root of aerobic metabolism in action, which means burning the 2 carbon atoms in acetic acid.
First things first – let’s just see how carbon atoms burn – by way of one of the most spectacular demonstrations ever staged and documented in human history.
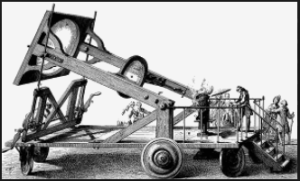
In the 1770’s Lavoisier burned a diamond via solar power to demonstrate it was just another form of carbon that combusted like coal when very highly heated. As a combustible fuel carbon atoms in a diamond combine with O2 in air. So the most simple ‘machine’ formula of combusting carbon with no other element involved is: C + O2 –> CO2 + Heat
To round out your thinking on what Lavoisier knew about igniting a diamond and other carbon based fuels, realize practically any combustible fuel will spontaneously ignite when heated to it’s ignition temperature. For example charcoal ignites at 716 °F, paper at 451°F, wood at 572 °F, and diamonds at 1652 °F.
‘Burning’ food or fuel is a generic description for combusting carbon atoms in a specific fuel such as wood, dried milk, propane, gasoline, coffee, diamonds, coal, etc.
The carbon in dried milk and coffee beans can even be burned to run a train.
Tap or click the image to dismiss or recover the captions.
C + O2 –> CO2
So far, I’ve shown you combusting food in either a machine locomotive or a human is simply a matter of combining carbon with oxygen to make CO2 – which humans came first came to know actually happens in humans around 1770.

Lavoiser’s wife Marie-Anne Paulze Lavoisier – seated right – illustrated herself and a 1770’s respiration experiment demonstrating ‘capturing carbon atoms’ in the CO2 expired. It was known oxygen combined with carbon in the body, but not known how it happened microscopically in cells.
By 1906 technology had advanced to measure the carbon atoms eaten from food – by capturing the CO2 expired.

By 1906, it was completely understood aerobic metabolism, or cellular respiration, required cells to break down primarily fat as the main fuel for the body (as carbon chains) into acetic acid (a shorter 2-carbon chunk fuel).
Carbon is the ultimate fuel for running internal combustion machines and humans.
However, unlike a machine’s ability to use and ignite most any carbon fuel the body burns only acetic acid – which requires breaking down mainly fat and carbohydrates – but sometimes protein or ketones into acetic acid. None of other stuff burns, they just provide the carbon atoms in different forms, which must be turned into acetic acid.
I just mentioned above, fat and carbohydrates break down into acetic acid. Let’s see how this works.
To visualize the primary source of food that breaks down into acetic acid as straightforward as possible – as it occurs in the body – I created an 8-second long clip showing the reason exactly why this source breaks directly down into acetic acid with no muss, fuss, or numerous biological conversions needed to do it.
There you have it. The clip actually showed connecting 5 acetic acids together into chains. Just reverse the process you just witnessed and the same 5 acetic acids are reproduced.
The fuel for aerobic metabolism is continuously supplied primarily by breaking chains of acetic back into single units of acetic acid.
Now here’s the kicker: Every type of fat in nature, e.g. olive oil, butter, lard, fish oil, etc. are made of chains of acetic acid. Let that sink in for a moment.
Again, all fat is made by connecting many acetic acids together “two carbon-chunks” at a time into a chain called a fatty acid. The lengths of the chain will be 4, 6, 8, etc carbon atoms long – up to 28 carbon atoms – dependent on the number of acetic acids connected.
Now, because all fat in nature is made of 2, 4, 6, etc. carbon backboned units called fatty acids – this explains why fatty acids break right back directly down into acetic acid with no muss and no fuss.
Thus, it is the continuous breakdown of food, but mainly fatty acids into acetic acid, which keeps us ‘breathing’ at the cell level.
Note: Carbohydrates also breakdown into acetic acid, but they do so only partially and not directly like fat… if they do. We’ll examine this at a different time.
Aerobic metabolism essentially reverses the construction of fatty acids to ‘spit out’ acetic acid for combustion within cells.
Let’s see an example of breaking down a fatty acid into acetic acid. It is the complete structure of a very commonly eaten fatty acid – made out of 9 acetic acids – thus making a total of 18 carbon atoms chained together.
Notice each end of the fatty acid is acetic acid split in half and placed on on each end – which I color coded.
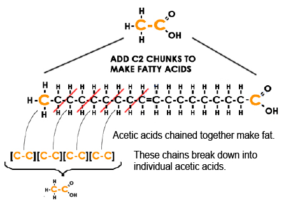
The 18 carbon length fatty acid is oleic acid. Oleic acid is the primary component of quality olive oil and lard.
As shown by the red slashes – four 2-carbon segments of oleic acid break down directly into four individual acetic acid molecules – indicated by four colored [C-C] pieces.
I excluded the oxygen and hydrogen atoms for practical purposes to showcase how “the making and breaking” of fatty acids is primarily a matter of counting by two’s in terms of carbon chunks; for the sake of simplicity the focus should only be on the carbon atoms.
- Using the basic fundamental of counting by two’s: we could say acetic acid is C2 because it has 2 carbon atoms.
- Hence, we could label the fatty acids found in the following foods this way: Butter (C4); Coconut oil (C10), and all fats and oils as C2, C4, C6, etc.
In fact, biochemists label fatty acids by the method above and I think it’s the way nutritionists should learn it, although most have no idea how fatty acids construct fat and why it matters in terms of understanding fat, calories, metabolism, and health.
At any rate, dependent on the length of the chain, fatty acids are classified as short, medium, or long – ranging from 4 to 28 total carbon atoms, pieced together by acetic acids.
Again, ALL fat is made out of acetic acid; it is the basic building block for butter, coconut oil, olive oil, and all fats in nature. You can easily tell how many acetic acids will result from a fatty acid’s length or number of carbon atoms – (chain length is one key for learning health properties and functions of fats, which we examine another time).
Returning to oleic acid splitting in the graphic, notice each split into acetic acid occurs at each second carbon atom across the chain from the left. Since beta is the second letter in the Greek alphabet, the process of making aerobic fuel out of fat is called beta oxidation.

Beta Oxidation = Breaking down fatty acids to make acetic acid.
SUMMARY
Respiration is a form of slow combustion in a cell – where vinegar burns to produce CO2, Water, and Heat.
At rest and low to moderate intensity levels fatty acids carried in blood, stored in muscles, and stored in body fat are the primary calories burned – after reducing to acetic acid.
Acetic acid burns in the mitochondria and a cell respires. Drawn below by a student, day 2 of class in 2013.
Carbohydrates do not breakdown directly into acetic acid. In fact they breakdown mostly into a totally different substance dependent on how you use your body.
1. Butter, Butter, Butter: The Physiology of Adventure Diets
This section of the lecture is a slide show – featuring a seminar I present annually during the Outdoor Adventure Expo at Midwest Mountaineering – titled Butter, Butter, Butter.
The primary objective of this lecture is to visualize the essential anatomical parts of the body’s aerobic system from a macro view down to the microscopic level where the action of metabolism finally takes place. The secondary objective is to see why butter and vinegar are essentially the two most simple aerobic fuels and know what really burns in cells.
The picture below is a simplified version of the whole.
Read the remaining sections before class so you are clear now on the visuals and basic knowledge you must know for the quiz or assignment.
The slide show graphics definitely require explanation to understand, especially regarding the relationship between butter/vinegar and aerobic metabolism. So you absolutely must attend this lecture.
Click here to view the slide show I present in class now if you wish.
Butter, Butter, Butter – Fats You Really Should be Eating
 Calories in are not equal to body heat out. Ever get cranky on the trail and wonder how your diet affects your mood and performance? Learn the biochemical differences between butter, lard, olive and fish oils and how weather and diet influence the thermodynamics of metabolism down to the cell level.
Calories in are not equal to body heat out. Ever get cranky on the trail and wonder how your diet affects your mood and performance? Learn the biochemical differences between butter, lard, olive and fish oils and how weather and diet influence the thermodynamics of metabolism down to the cell level.
Learn how to optimize your adventure by feeding your body properly and learn how to pack the appropriate provisions for your next trek.
Recap Week 10 and Overview Week 11, 2017
Recap Week 10
Clarified Butter = Ghee: Because it has been fermented there is negligible lactose (much less than a gram). Lactose intolerant people can ingest 8g and remain unaffected.
Lymph: What is it spatially/anatomically – around the cells, vessels, and nodes?
Match Stick: Look at Week 3 chart: Glycogen Depletion Rates
Billion Year Old Carbon:
Overview Week 11, 2017
O2 consumption is a simple concept. Carbon burns.
Graphing VO2 is even simpler. Move, mover faster – and the graph is essentially a straight line until ‘the fire’ reaches its maximum rate of burning. At this point – no additional power is produced by oxidizing carbon.
Handout
Plant Respiration & Synergy of Energy Transformation
Props
Matchstick
Candle
Why does the wick burn extremely slowly?
What is actually burning mostly?
What three states of matter – of the main substance burning – do we see simultaneously?
What is the equivalent reaction in your body?
Where does this reaction occur specifically?
What are the reactants and what are the products of the reaction?
What ‘runs out’ in cave or sealed space when we suffocate?
Why would a fast burning explosive kill you by not damaging you physically?
Videos and/or Additional Reading
What burns when you light a candle?
http://home.howstuffworks.com/question267.htm
http://thehappyscientist.com/science-experiment/whats-burning
Thermodynamics of Weight Loss:
Thermodynamics of Weight Loss Diets
Lulu Hunt Peters: Diet & Health
Death by instantaneous removal of oxygen from air:
More on oxygen:
Molecules, magic, and forgetful fruitflies; the supernatural science of medical gas research

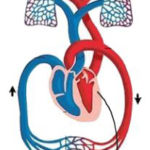


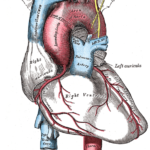
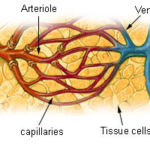

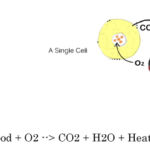
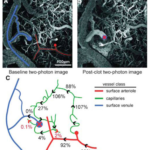


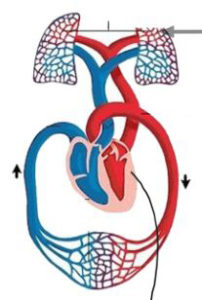
Aerobic metabolism is a way for your cells to convert fat, carbohydrate and sometimes protein into energy, but only in the presence of oxygen.
Yes indeed.