Life in the Cell Begins at the Edge of Death, Part 6
Part 6: Metabolism at the Atomic Level… Burning Carbon is IT
Parts 6, 7, and 8 are monumental. This is the first step to rebuild your entire picture of what food really is and how the body works. At this point I feel I haven’t shown you much besides what you knew already, i.e. how fire works in nature or in the body.
Everything up to this point was just a setup for the next few parts, which show you a new, more complete way to understand food and metabolism.
All fuels sources – acetic acid, charcoal, wood, milk, gasoline, diamonds, and foods – are made principally out of carbon. And since the single fuel that combines with oxygen is C:
Any fuel burning is carbon atoms combusting.
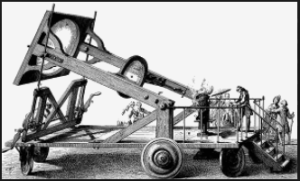
Burning a diamond with solar power represents the absolute ‘cleanest’ version of carbon atoms burning – as demonstrated in the 1770’s by Antoine Lavoisier. He burned a diamond to prove it burns just like coal and to demonstrate diamonds are super compressed carbon – or an allatrope of carbon.
Burning either form of just pure carbon (diamonds or coal), we have:
C + O2 –> CO2
Carbon fuels combust either openly in air or within the specific machine capable of combining a specific carbon based fuel molecule with oxygen. Here are three examples:
1) A train’s firebox can burn a variety of different carbon fuel molecules found in wood, milk, or coal.
2) A butane lighter can specifically burn butane gas, but not milk, wood, or coal. Similarly, your body can’t burn just anything like tossing anything in a bonfire; your cells need a specific fuel.
3) Your cells are a specific machine and acetic acid is the specific fuel burning in your cells.
Now, when acetic acid burns in your body – the 2 carbon atoms in it combine with oxygen – so we see:
C + O2 (twice) –> CO2, CO2 …. forever as long as you breathe.
If a human is an aerobic machine where C combines with O2, then we should see the CO2 coming out of our body.
We do, when we trap and save it all in a bottle or other device.
Besides burning a diamond, Lavoisier collected CO2 gas in another 1770’s experiment, proving metabolism is a form of combustion. For the record, his wife Marie-Anne illustrated his experiments.
So, within your body, carbon is constantly combining with oxygen to produce CO2 gas.
Deep inside your body, oxygen combines with fuel to produce carbon dioxide. CO2 gas streams out of the body – first out of your cells into your venous blood stream, then out of your lungs and out your mouth.
So what’s the difference between machines and humans? Why can’t humans burn wood, coal, or milk directly?
‘Junk’ carbon like wood, coal, or dried milk must be heated up hundreds of degrees in order to combust. For example, wood ignites around 300 degrees, charcoal at 660, and a diamond at 900 – all in degrees Farenheit. The reason dried milk, coal, or wood burn readily in a train is simply because extreme heat ‘trips the trigger’ to cause carbon atoms to react/combine with oxygen. That’s why we light a match; we use a fire or high heat to start a fire. By extension, burning a diamond requires even more heat than coal or wood, but burns the same way, given enough heat.
Our bodies are different. Our cells are not designed to burn ‘junk’ carbon. And besides, extreme heat would burn us, so our cells must use a different way to burn a fuel to produce power. Now, acetic acid indeed burns in our body – but at a lower temperature. In a nutshell, this is made possible by enzymes in our cells which lower the temperature required to combust it. Hence, your body temp stays around 98.6 degrees Farenheit as opposed to say 451… the temperature where paper ignites.
Main Points of Burning Carbon
- All food and ‘junk’ fuel is made of large carbon based molecules.
- Food breaks down into acetic acid, only to burn up instantly the moment it is produced.
- The entire body itself is a gigantic aerobic machine where all CO2 produced and blown out is just the collective sum of millions of ‘tiny aerobic machines’ combining O2 with acetic acid.
It’s time to look at the combustion in a single cell.
Next Part 7: A single Cell’s Mitochondria is the Aerobic Machine of Your Body